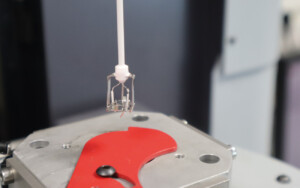
An overview of Simultaneous Thermal Analysis
Simultaneous thermal analysis measures the thermal stability of materials, and provides information about their reactivity or composition. It is sometimes called STA (for simultaneous thermal analysis). It is also often referred to as TG-DSC or TG-DTA as this technique combines thermogravimetry with DTA or DSC methods.
STA – Introduction to Simultaneous Thermal Analysis
Simultaneous thermal analysis is a technique based on both measurements of mass variation of a material sample to be characterized and of temperature or heat flow differences between this material sample and an inert reference.
These measurements are performed simultaneously when the sample is subjected to a given temperature or to a temperature variation program.
The instrument used to apply this technique is mainly composed of :
- A balance capable of continuously measuring the of mass variation of the sample with respect to its initial mass. The measured variations being often very weak, one generally speaks about microbalance.
- A sensor to carry the sample to be measured and the reference material. It incorporates a differential thermocouple, which continuously measures their temperature difference in the case of TG-DTA, or their heat flow difference in the case of TG-DSC. This sensor, often called a rod because of its thin and long shape, is attached to the balance.
- An oven, in which the sample is placed during the experiment. This oven is equipped with a temperature control system that allows it to heat, cool, and maintain the temperature of the sample according to a profile programmed by the user.
- A gas management system that allows to control the composition and the renewal of the atmosphere around the sample. Depending on the desired application, the nature of the gas(es) used can be chosen to generate an inert atmosphere, or on the contrary, a reactive atmosphere when a specific reaction of the sample is to be analyzed.
Depending on their level of sophistication and performance, simultaneous thermal analyzers can cover the needs of research & development, quality control or education.
STA and mass variation measurements
The analysis of the mass variation as a function of time or temperature allows to characterize the phenomena leading to mass losses: decompositions, water or solvent losses, oxides reductions, etc. It also enables the characterization of mass gains linked to adsorptions or oxidations.
This analysis helps according to the case to :
- establish and/or compare the thermal stability of various materials, i.e. their ability to withstand high temperatures.
- obtain information on the composition of these materials, such as the ash and filler content, their water content, organic matter, etc.
- characterize gas-solid or gas-liquid reactions such as oxidations or adsorptions by studying the quantities and rates of reaction.

STA and temperature measurements
When the sample is heated in the STA and undergoes a transformation, its difference in temperature or heat flow with the inert reference increases. It then returns to a baseline value after the transformation is complete. Thus, analysis of a curve representing these signals as a function of temperature can characterize the transformation.
If the analysis of the variation of mass versus temperature can determine temperatures of decomposition, desorption, dehydration, etc., the curves of temperature difference or heat flow allows to measure the temperatures of melting, crystallization, and phase change (solid-solid transformations) of materials.
Thus the combination of these measurements allows a more complete characterization than thermogravimetry alone, or than DTA or DSC alone.

TG-DSC and heat measurements
In the specific case of the TG-DSC, a simple mathematical treatment (an integration) of the curve representing the difference in heat flow as a function of time makes it possible to determine the heat of the measured thermal effect.
This mathematical treatment, which can be easily performed by any user, allows to determine the heats of fusion, crystallization, reaction, decomposition and phase change (solid-solid transformations) of materials.
The heat flow difference curve also makes it possible to distinguish endothermic effects (which absorb heat: e.g. melts, transitions to a less stable phase) from exothermic effects (which emit heat: e.g. crystallizations, most decompositions).
These measurements are for example important for estimating the heat to be supplied to or removed from the material before using it in an industrial process.